Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the number of moles of chemical units represented by 9.03x10^24? and how do i show work? (dumb it down )
Answers: 1

Chemistry, 22.06.2019 06:30
The minerals found in bones are deposited by living cells called
Answers: 1

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2
You know the right answer?
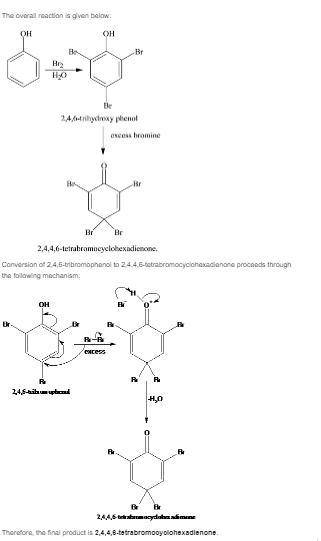
Treatment of phenol with excess aqueous bromine is actually more complicated than expected. a white...
Questions

Biology, 14.01.2020 19:31

Social Studies, 14.01.2020 19:31










Computers and Technology, 14.01.2020 19:31

Computers and Technology, 14.01.2020 19:31





Social Studies, 14.01.2020 19:31