Chemistry, 15.01.2020 05:31 hannahcolleen4895
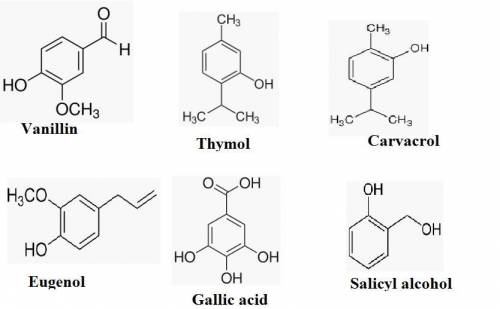
The iupac rules permit the use of common names for a number of familiar phenols and aryl ethers. these common names are listed here along with their systematic names. write the structure of each compound.
a. vanillin(4-hydroxy-3-methoxybenzald ehyde): a component of vanilla bean oil, which contributes to its characteristic flavor.
b. thymol (2-isopropyl-5-methylphenol): obtained from oil of thyme.
c. carvacrol (5-isopropyl-2-methylphenol): present in oil of thyme and marjoram.
d. eugenol (4-allyl-2-methoxyphenol): obtained from oil of cloves.
e gallic acid (3,4,5-trihydroxybenzoic acid): prepared by hydrolysis of tannins derived from plants.
f. salicyl alcohol (o-hydroxybenzyl alcohol): obtained from bark of poplar and willow trees.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 20:00
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3
You know the right answer?
The iupac rules permit the use of common names for a number of familiar phenols and aryl ethers. the...
Questions


Health, 06.02.2021 14:00




Physics, 06.02.2021 14:00


Mathematics, 06.02.2021 14:00

Law, 06.02.2021 14:00


Mathematics, 06.02.2021 14:00

Mathematics, 06.02.2021 14:00


Mathematics, 06.02.2021 14:00

Mathematics, 06.02.2021 14:00





Mathematics, 06.02.2021 14:00

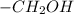 group is linkedto benzene ring and in respect to that hydroxyl group is present at ortho position of the ring.
group is linkedto benzene ring and in respect to that hydroxyl group is present at ortho position of the ring.