
Chemistry, 15.01.2020 03:31 angeljohnson2081
Sodium hydrogen carbonate nahco3, also known as sodium bicarbonate or "baking soda", can be used to relieve acid indigestion. acid indigestion is the burning sensation you get in your stomach when it contains too much hydrochloric acid hcl, which the stomach secretes to digest food. drinking a glass of water containing dissolved nahco3 neutralizes excess hcl through this reaction: hcl(aq)+nahco3(aq)→nacl(aq)+h2o(l)+ co2(g)
the co2gas produced is what makes you burp after drinking the solution. suppose the fluid in the stomach of a woman suffering from indigestion can be considered to be 200.ml of a 0.089m hcl solution. what mass of nahco3 would she need to ingest to neutralize this much hcl? be sure your answer has the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution? a. 3.88 m, b. 1.03 m, c. 1.5 m, d. 15.5 m
Answers: 3

Chemistry, 21.06.2019 22:30
Joseph has hypothesized that sound travels in waves. if he were following the scientific method, what should he do next? a. ask a question. b. test the hypothesis. c. study the results. d. tell other scientists about his hypothesis.
Answers: 1

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1
You know the right answer?
Sodium hydrogen carbonate nahco3, also known as sodium bicarbonate or "baking soda", can be used to...
Questions










Social Studies, 28.06.2019 15:00



History, 28.06.2019 15:00

Mathematics, 28.06.2019 15:00


English, 28.06.2019 15:00


English, 28.06.2019 15:00


Mathematics, 28.06.2019 15:00

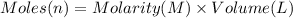
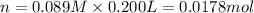 of HCL
of HCL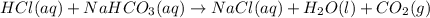
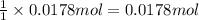 of sodium bicarbonate
of sodium bicarbonate

