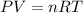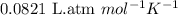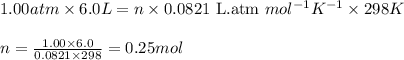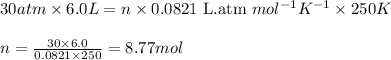The average lung capacity of a human is 6.0l.
how many moles of air are in your lungs, when you are in the following situations?
(a) at sea level where t=298 k, and p = 1.00 atm.
(b) on top of mt everest where t = 200 k and p = 0.296 atm.
(c) trying to escape from a sunken submarine where t = 250 k and p = 30 atm.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3
You know the right answer?
The average lung capacity of a human is 6.0l.
how many moles of air are in your lungs, when yo...
how many moles of air are in your lungs, when yo...
Questions


English, 11.02.2020 22:28





Health, 11.02.2020 22:28



Computers and Technology, 11.02.2020 22:28


English, 11.02.2020 22:29

Mathematics, 11.02.2020 22:29

Mathematics, 11.02.2020 22:29


English, 11.02.2020 22:29

Social Studies, 11.02.2020 22:29

Biology, 11.02.2020 22:29

Mathematics, 11.02.2020 22:29