Chemistry, 14.01.2020 23:31 morenodonaldo762
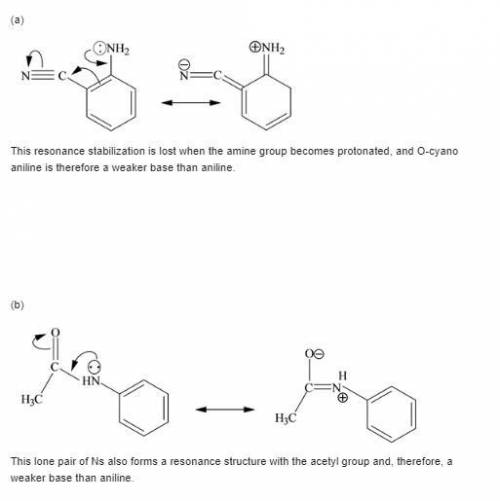
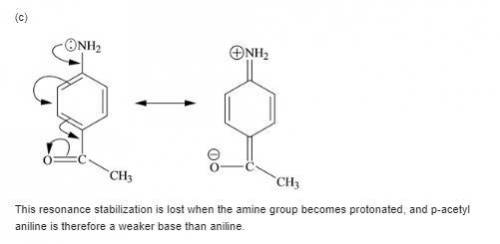
Each of the following is a much weaker base than aniline. present a resonance argument to explain the effect of the substituent in each case.
a. o-cyanoaniiine
b. p-aminoacetophenone
c. c6h5nhcch3
||
o

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1

Chemistry, 23.06.2019 07:30
Assume that 13.5 g solid aluminum (al) react with hcl to produce solid aluminum chloride (alcl3) salt and gaseous hydrogen (h2) at standard temperature and pressure.
Answers: 1

Chemistry, 23.06.2019 10:30
Which of the following characteristics are true of enzymes? check all that apply. a.)the structure of an enzyme can change if conditions change. b.)a single enzyme can normally catalyze a wide variety of reactions under many conditions. c.)enzymes are found only in nonliving systems. d.)enzymes allow living things to regulate body conditions through feedback mechanisms. e.)enzymes bind to specific substrates in specific ways. f.)enzymes increase the rate of reaction. g.)when shown in energy-reaction diagrams, enzymes represent the higher activation energy.
Answers: 1
You know the right answer?
Each of the following is a much weaker base than aniline. present a resonance argument to explain th...
Questions

English, 24.11.2019 00:31


History, 24.11.2019 00:31



Mathematics, 24.11.2019 00:31



Health, 24.11.2019 00:31

Biology, 24.11.2019 00:31

Mathematics, 24.11.2019 00:31

Biology, 24.11.2019 00:31



Mathematics, 24.11.2019 01:31

Social Studies, 24.11.2019 01:31



Mathematics, 24.11.2019 01:31

English, 24.11.2019 01:31