A2.241-g sample of nickel reacts with oxygen to form 2.852 g of the metal oxide.
calculate the...

Chemistry, 14.01.2020 05:31 JAYDENJONES0111
A2.241-g sample of nickel reacts with oxygen to form 2.852 g of the metal oxide.
calculate the empirical formula for oxide.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Afamily is one another name for a group on the table of elements.
Answers: 1

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

You know the right answer?
Questions

Mathematics, 24.03.2021 20:00

Chemistry, 24.03.2021 20:00

Mathematics, 24.03.2021 20:00

Physics, 24.03.2021 20:00

History, 24.03.2021 20:00

History, 24.03.2021 20:00


Mathematics, 24.03.2021 20:00

Mathematics, 24.03.2021 20:00


Social Studies, 24.03.2021 20:00


Mathematics, 24.03.2021 20:00




Mathematics, 24.03.2021 20:00



English, 24.03.2021 20:00

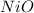
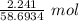 = 0.03818 mol
= 0.03818 mol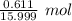 = 0.03818 mol
= 0.03818 mol

