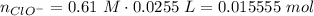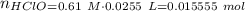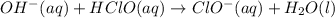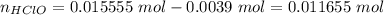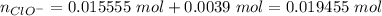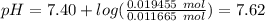Abuffer solution with a volume of 0.0255 l consists of 0.61 m hypochlorous acid (hclo), a weak acid, plus 0.61 m potassium hypochlorite (kclo). the acid dissociation constant of hypochlorous acid, ka, is 4.0 x 10^−8.
1. determine the ph of the buffer solution after the addition of 0.0039 mol rubidium hydroxide (rboh), a strong base. (assume no change in solution volume.)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
You know the right answer?
Abuffer solution with a volume of 0.0255 l consists of 0.61 m hypochlorous acid (hclo), a weak acid,...
Questions




Geography, 13.11.2020 17:10








Computers and Technology, 13.11.2020 17:10




Mathematics, 13.11.2020 17:10

Mathematics, 13.11.2020 17:10



![pH = pK_a + log(\frac{[A^-]}{[HA]})](/tpl/images/0453/4960/e07d5.png)
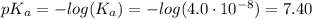
![[A^-] = [ClO^-]](/tpl/images/0453/4960/01383.png)
![[HA] = [HClO]](/tpl/images/0453/4960/a69d6.png)