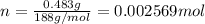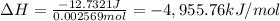Chemistry, 13.01.2020 19:31 nayelimoormann
A0.483-g sample of nonanedioic acid (c9h16o4) is burned in a bomb calorimeter and the temperature increases from 24.70 °c to 27.20 °c. the calorimeter contains 1.01×103 g water and the bomb has a heat capacity of 867 j/°c. based on this experiment, calculate δe (kj/mol) for the combustion reaction.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 19:40
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests.which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3

Chemistry, 23.06.2019 04:40
Equal numbers of moles of he(g), ar(g), and ne(g) are placed in a glass vessel at room temperature. if the vessel has a pinhole-sized leak, which of the following will be true regarding the relative values of the partial pressures of the gases remaining in the vessel after some of the gas mixture has effused?
Answers: 1

Chemistry, 23.06.2019 05:00
How many atomic mass units are equal to 1.672×10−24 g of protons?
Answers: 3
You know the right answer?
A0.483-g sample of nonanedioic acid (c9h16o4) is burned in a bomb calorimeter and the temperature in...
Questions


Mathematics, 07.07.2019 04:10

Computers and Technology, 07.07.2019 04:10




Business, 07.07.2019 04:20

History, 07.07.2019 04:20






Spanish, 07.07.2019 04:20

Mathematics, 07.07.2019 04:20


Spanish, 07.07.2019 04:20



Mathematics, 07.07.2019 04:20

![q=[q_1+q_2]](/tpl/images/0453/1616/341bc.png)
![-q=[c_1\times \Delta T+m_2\times c_2\times \Delta T]](/tpl/images/0453/1616/37582.png)
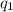 = heat absorbed by the calorimeter
= heat absorbed by the calorimeter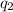 = heat absorbed by the water
= heat absorbed by the water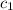 = specific heat of calorimeter =
= specific heat of calorimeter = 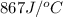
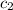 = specific heat of water =
= specific heat of water = 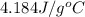
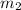 = mass of water =
= mass of water = 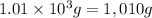
 = change in temperature = 27.20 °C - 24.70 °C =2.5°C
= change in temperature = 27.20 °C - 24.70 °C =2.5°C![-q=[(867 J/^oC\times 2.5 ^oC)+(1,010\times 4.184J/g^oC\times 2.5^oC)]](/tpl/images/0453/1616/a0608.png)
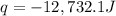
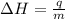
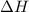 = enthalpy change = ?
= enthalpy change = ?