Urgent plese and you
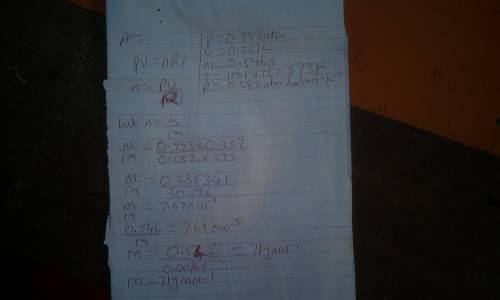
an ideal gas sample weighing .546 g at 100 degrees celsius and .993 atm...

Chemistry, 11.01.2020 03:31 monsterduckgoose
Urgent plese and you
an ideal gas sample weighing .546 g at 100 degrees celsius and .993 atm has a volume of .237 l. determine the molar mass of the gas.
a. 71.3 g/mol
b. 143 g/mol
c. 19.1 g/mol
d. 0140 g/mol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 17:30
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2
You know the right answer?
Questions


Chemistry, 17.12.2020 01:00

Mathematics, 17.12.2020 01:00

Health, 17.12.2020 01:00



Mathematics, 17.12.2020 01:00


Physics, 17.12.2020 01:00


Computers and Technology, 17.12.2020 01:00

Mathematics, 17.12.2020 01:00

Mathematics, 17.12.2020 01:00

Mathematics, 17.12.2020 01:00

Computers and Technology, 17.12.2020 01:00

History, 17.12.2020 01:00

Chemistry, 17.12.2020 01:00

Mathematics, 17.12.2020 01:00


Biology, 17.12.2020 01:00