
Chemistry, 10.01.2020 06:31 aidanwindsor1738
Asource of zinc metal can be zinc ore containing zinc(ii) sulfide. the ore is roasted in pure oxygen to produce the oxide and then reduced with carbon to form elemental zinc and carbon monoxide. 2 zns + o2 2 zno + 2 so2 zno + c zn + co a crucible containing a sample of 0.50 mol zns was roasted in pure oxygen, then reduced with 1.00 mol carbon. what mass remained in the crucible after cooling?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 22:00
11) burning your hand when accidentally touching a hot plate is an example of which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 2

You know the right answer?
Asource of zinc metal can be zinc ore containing zinc(ii) sulfide. the ore is roasted in pure oxygen...
Questions








Social Studies, 04.01.2020 05:31

English, 04.01.2020 05:31





Social Studies, 04.01.2020 05:31






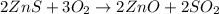 ..[1]
..[1]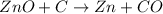 ..[2]
..[2]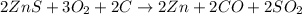 ..[3]
..[3]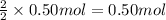 of Zn.
of Zn.

