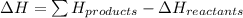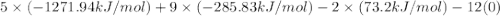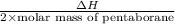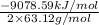Pentaborane-9,
, is a colorless, highly reactive liquid thatwill burst into flame
when exposed to oxygen. the reactionis:
calculate the kilojoules of heat released per gram of
thecompound reacted with oxygen. the standard enthalpy of formation
ofb5 h9 is 73.2 kj/mol.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
You are performing an experiment in a lab to attempt a new method of producing pure elements from compounds. the only problem is that you do not know what element will form. by your previous calculations you know that you will have 6.3 moles of product. when it is complete, you weigh it and determine you have 604.4 grams. what element have you produced?
Answers: 1

Chemistry, 22.06.2019 03:30
If you have 5.25 grams of methane (ch4), how many grams of co2 will you produce ?
Answers: 1

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3
You know the right answer?
Pentaborane-9,
, is a colorless, highly reactive liquid thatwill burst into flame
when...
, is a colorless, highly reactive liquid thatwill burst into flame
when...
Questions




Mathematics, 27.05.2021 03:30


Mathematics, 27.05.2021 03:30


Mathematics, 27.05.2021 03:30

Mathematics, 27.05.2021 03:40


Mathematics, 27.05.2021 03:40

Geography, 27.05.2021 03:40






Mathematics, 27.05.2021 03:40

Mathematics, 27.05.2021 03:40

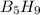 ) is a colorless highly reactive liquid that will burst into flames when exposed to oxygen.the reaction is:
) is a colorless highly reactive liquid that will burst into flames when exposed to oxygen.the reaction is:
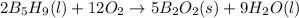
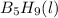 ,
, 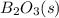 , and
, and 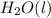 are 73.2, -1271.94, and -285.83 kJ/mol, respectively.
are 73.2, -1271.94, and -285.83 kJ/mol, respectively.