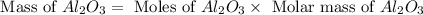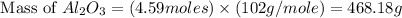Chemistry, 09.01.2020 06:31 josephmelichar777
The reaction betwen aluminum and iron (iii) oxide can
generatetemperatues approaching 3000 degrees celsius and is used in
weldingmetals:
2al + fe2o3
> al2o3 + 2 fe
in one process, 124 g of al are reacted with 601 g
offe2o3. calculate the mass in grams
ofal2o3 formed.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 16:00
How do dying stars contribute to the formation of planets
Answers: 1
You know the right answer?
The reaction betwen aluminum and iron (iii) oxide can
generatetemperatues approaching 3000 deg...
generatetemperatues approaching 3000 deg...
Questions

Mathematics, 05.05.2020 14:29

Mathematics, 05.05.2020 14:29



History, 05.05.2020 14:29

Spanish, 05.05.2020 14:29


Mathematics, 05.05.2020 14:29


Mathematics, 05.05.2020 14:29


Social Studies, 05.05.2020 14:29

History, 05.05.2020 14:29


History, 05.05.2020 14:29



History, 05.05.2020 14:29

Biology, 05.05.2020 14:29

 formed will be, 468.18 grams.
formed will be, 468.18 grams.  = 601 g
= 601 g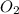 .
.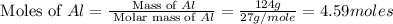
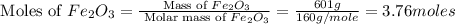
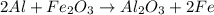
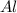 react with 1 mole of
react with 1 mole of 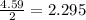 moles of
moles of 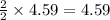 moles of
moles of