If the vapor pressure of ethanol at 34.7degree c is 100
mmhg, and its heat of vaporization is...

Chemistry, 09.01.2020 06:31 loanyst99111
If the vapor pressure of ethanol at 34.7degree c is 100
mmhg, and its heat of vaporization is 38.6 kj/mol. at what
temperaturewould ethanol boil at sea level?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1

Chemistry, 23.06.2019 01:30
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1

Chemistry, 23.06.2019 03:30
Which of the following describes the entropy change as a solution is made from a liquid and solid
Answers: 1
You know the right answer?
Questions

Mathematics, 04.07.2019 10:10


English, 04.07.2019 10:10

Biology, 04.07.2019 10:10

Mathematics, 04.07.2019 10:10



Mathematics, 04.07.2019 10:10


Social Studies, 04.07.2019 10:20


Mathematics, 04.07.2019 10:20

Mathematics, 04.07.2019 10:20

Mathematics, 04.07.2019 10:20

Social Studies, 04.07.2019 10:20

History, 04.07.2019 10:20


Mathematics, 04.07.2019 10:20

Health, 04.07.2019 10:20

Mathematics, 04.07.2019 10:20

![\frac{ΔvapH}{R}([tex]\frac{1}{T1}](/tpl/images/0448/1509/1cff8.png) -
-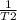 )
)

