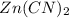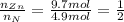Chemistry, 09.01.2020 03:31 twalters88
An unknown compound has the following chemical formula: zn(cn)x where x stands for a whole number. measurements also show that a certain sample of the unknown compound contains 9.7 mol of nitrogen and 4.9 mol of zinc. write the complete chemical formula for the unknown compound.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 12:00
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1
You know the right answer?
An unknown compound has the following chemical formula: zn(cn)x where x stands for a whole number....
Questions

Physics, 24.06.2019 01:00

Mathematics, 24.06.2019 01:00



History, 24.06.2019 01:00


Chemistry, 24.06.2019 01:00

Mathematics, 24.06.2019 01:00

Mathematics, 24.06.2019 01:00





Computers and Technology, 24.06.2019 01:00


Mathematics, 24.06.2019 01:00