Chemistry, 08.01.2020 09:31 lisa123465
1. how many moles of nitrogen monoxide can be made using 5.0 moles of oxygen in
the following composition reaction?
n2 + o2 -> no
2. the neutralization of an acid with a base is a double replacement reaction in which a salt and water are formed. if you start with 25 moles of hcl and neutralize it with
naoh how many moles of nacl will be formed?
hcl + naoh->
3. a car burns gasoline (octane – c8h18) with oxygen. if you drive to salt lake and
burn 150 moles of octane how many moles of carbon dioxide are you producing?
4. if 25 gram of magnesium combines with oxygen in a composition reaction, how
many moles of magnesium oxide will be formed?
mg + o2 -> mgo
5. lithium reacts with water in a single replacement reaction. how many moles of
hydrogen gas a produced by 10 grams of lithium?
li + hoh ->
6. barium chloride reacts with sodium sulfate in a double replacement reaction. how
many grams of barium chloride are required to react with 5 moles of sodium sulfate?
7. magnesium carbonate when heated decomposes to form magnesium oxide and carbon dioxide. how many grams of magnesium oxide will be formed if 20 grams of
magnesium carbonate are heated?
mgco3 -> mgo + co2
8. if 70 grams of gold iii chloride decomposes into its elements, how many grams of
gold will be produced?
aucl3 -->
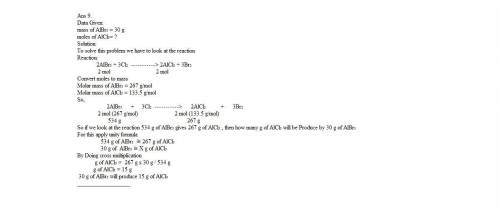
9. chlorine is more reactive element than bromine, thus chlorine will replace bromine in compound through a single replacement reaction. if 30 grams of aluminum bromide react with chlorine in this fashion how many grams of aluminum chloride will be formed?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Problem #3 (ch. 1, problem 15)the ideal gas law provides one way to estimate the pressure exerted by a gas on a container. the law isí‘ťí‘ť=푛푛푛푛푛푛푉푉more accurate estimates can be made with the van der waals equationí‘ťí‘ť=푛푛푛푛푛푛푉푉â’푛푛푟푟â’푞푞푛푛2푉푉2where the term nb is a correction for the volume of the molecules and the term an2/v2is a correction for molecular attractions. the values of a and b depend on the type of gas. the gas constant is r, the absolutetemperature is t, the gas volume is v, and the number of moles of gas molecules is indicated by n. if n = 1 mol of an ideal gas were confined to a volume of v = 22.41 l at a temperature of 0â°c (273.2k), it would exert a pressure of 1 atm. in these units, r = 0.0826.for chlorine gas (cl2), a = 6.49 and b = 0.0562. compare the pressure estimates given by the ideal gas law and the van der waals equation for 1 mol of cl2 in 22.41 l at 273.2 k. what is the main cause of the difference in the two pressure estimates, the molecular volume or the molecular attractions?
Answers: 1

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3
You know the right answer?
1. how many moles of nitrogen monoxide can be made using 5.0 moles of oxygen in
the following...
the following...
Questions






Chemistry, 31.03.2020 01:57

Mathematics, 31.03.2020 01:57

History, 31.03.2020 01:57

Mathematics, 31.03.2020 01:57

Mathematics, 31.03.2020 01:57

Mathematics, 31.03.2020 01:57

Mathematics, 31.03.2020 01:57

Physics, 31.03.2020 01:57


History, 31.03.2020 01:57