
Chemistry, 06.01.2020 02:31 kayleetweedy1
In the final chemical equation, hf and o2 are the products that are formed through the reaction between h2o and f2. before you can add these intermediate chemical equations, you need to alter them by multiplying the
a. second equation by 2 and reversing the first equation.
b. first equation by 2 and reversing it.
c. first equation by (1/2) and reversing the second equation.
d. second equation by 2 and reversing it.
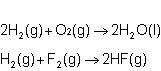

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 23.06.2019 04:31
Chemical engineering who specializes in negotiating for large purchases and instructing customers in use of the products are
Answers: 1

Chemistry, 23.06.2019 08:30
Benzonitrile (c6h5cn) is reduced to two different products depending on the reducing agent used. treatment with lithium aluminum hydride followed by water forms k, which has a molecular ion in its mass spectrum at 107 and the following ir absorptions: 3373, 3290, 3062, 2920, and 1600 cm-1. treatment with a milder reducing agent forms l, which has a molecular ion in its mass spectrum at 106 and the following ir absorptions: 3086, 2850, 2820, 2736, 1703, and 1600 cm-1. l shows fragments in its mass spectrum at m/z = 105 and 77. propose structures for k and l and choose an explanation for how this could be concluded.
Answers: 3
You know the right answer?
In the final chemical equation, hf and o2 are the products that are formed through the reaction betw...
Questions

Mathematics, 18.12.2020 20:30


Mathematics, 18.12.2020 20:30





History, 18.12.2020 20:30

Computers and Technology, 18.12.2020 20:30

Health, 18.12.2020 20:30


Social Studies, 18.12.2020 20:30


Biology, 18.12.2020 20:30

Mathematics, 18.12.2020 20:30



Mathematics, 18.12.2020 20:30


Health, 18.12.2020 20:30



