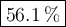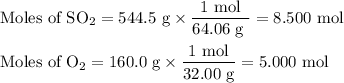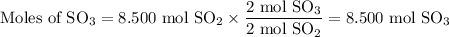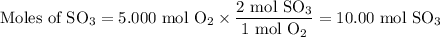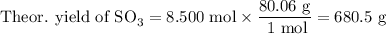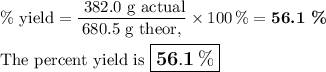Chemistry, 05.01.2020 19:31 johnandashley5p65r4a
What is the percent yield of the reaction below when 544.5 g so2 and 160.0g o2 produce 382.0 g so3? 2so2+o2+2so3

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
How much heat is released upon converting one mole of steam (18.0 g) from 100.0 ∘c to water at 25.0 ∘c? show work and constants, trying to figure out how it works. only given the heat capacity for steam and water so try to only use that
Answers: 1

Chemistry, 22.06.2019 00:00
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

Chemistry, 23.06.2019 02:00
The bohr model of the atom explained why emission spectra are discrete. it could also be used to explain the photoelectric effect. which is a correct explanation of the photoelectric effect according to the model?
Answers: 3
You know the right answer?
What is the percent yield of the reaction below when 544.5 g so2 and 160.0g o2 produce 382.0 g so3?...
Questions

Chemistry, 23.11.2020 23:50


Biology, 23.11.2020 23:50


English, 23.11.2020 23:50

History, 23.11.2020 23:50


Computers and Technology, 23.11.2020 23:50

Mathematics, 23.11.2020 23:50


Mathematics, 23.11.2020 23:50

Computers and Technology, 23.11.2020 23:50

Computers and Technology, 23.11.2020 23:50


History, 23.11.2020 23:50

Advanced Placement (AP), 23.11.2020 23:50


Mathematics, 23.11.2020 23:50