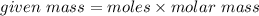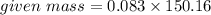Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Asolution has a ca2+ concentration of 0.049 m and an f- concentration is 0.147 m at equilibrium. the value of ksp for caf2 at 25°c is 4.0 x 10-11. will this solution form a precipitate? yes no
Answers: 3

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2
You know the right answer?
In an experiment, 9 g of aluminum react with 8 g of sulfur to form aluminum sulfide. calculate the g...
Questions


Mathematics, 20.04.2021 21:10

Mathematics, 20.04.2021 21:10


Mathematics, 20.04.2021 21:10

Mathematics, 20.04.2021 21:10


History, 20.04.2021 21:10



Mathematics, 20.04.2021 21:10

Mathematics, 20.04.2021 21:10

Mathematics, 20.04.2021 21:10

Mathematics, 20.04.2021 21:10

History, 20.04.2021 21:10

Physics, 20.04.2021 21:10



Mathematics, 20.04.2021 21:10

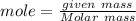
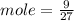
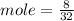
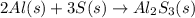
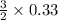 moles of S
moles of S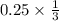
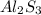 = 0.083 mole
= 0.083 mole