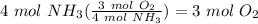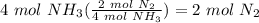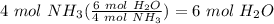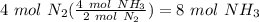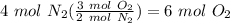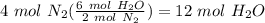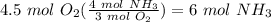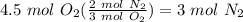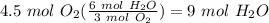Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 2

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1
You know the right answer?
Balance the following equation. then, given the moles of reactant or product below, determine the co...
Questions


Mathematics, 20.09.2020 18:01

English, 20.09.2020 18:01


Chemistry, 20.09.2020 18:01

Mathematics, 20.09.2020 18:01

Computers and Technology, 20.09.2020 18:01





Mathematics, 20.09.2020 18:01

Mathematics, 20.09.2020 18:01


Chemistry, 20.09.2020 18:01

Mathematics, 20.09.2020 18:01

Mathematics, 20.09.2020 18:01

History, 20.09.2020 18:01


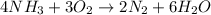 ". A complete explanation is below.
". A complete explanation is below.