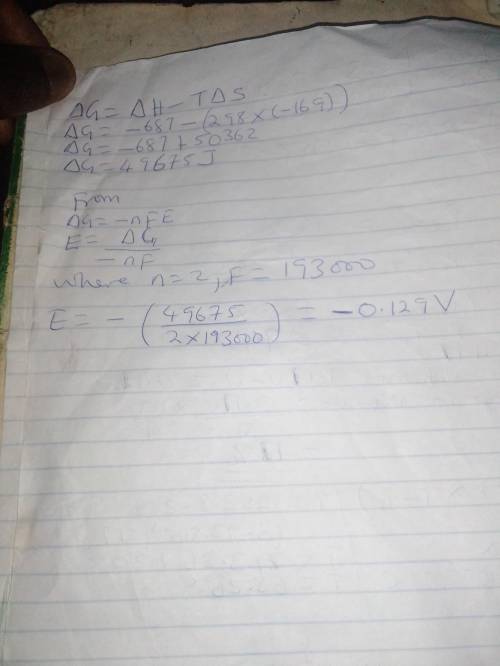Chemistry, 02.01.2020 22:31 Kiaraboyd9366
Calculate the standard cell potential at 25 ∘c for the reaction x(s)+2y+(aq)→x2+(aq)+2y(s) where δh∘ = -687 kj and δs∘ = -169 j/k .
express your answer to three significant figures and include the appropriate

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. initial mass and yield sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 1

Chemistry, 21.06.2019 22:30
Each of the following compounds contains a metal that can exhibit more than one ionic charge. provide systematic names for each of these compounds. (a) cr(clo3)6 (b) mo(cn)6 (c) cr2(so3)3 (d) v(clo2)2 (e) v(cn)5 (f) os(clo2)4
Answers: 3

Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1
You know the right answer?
Calculate the standard cell potential at 25 ∘c for the reaction x(s)+2y+(aq)→x2+(aq)+2y(s) where δh∘...
Questions


English, 21.05.2021 01:00

Mathematics, 21.05.2021 01:00


Mathematics, 21.05.2021 01:00

Mathematics, 21.05.2021 01:00

English, 21.05.2021 01:00

Mathematics, 21.05.2021 01:00

Mathematics, 21.05.2021 01:00


Mathematics, 21.05.2021 01:00


Social Studies, 21.05.2021 01:00


Physics, 21.05.2021 01:00


Business, 21.05.2021 01:00



Mathematics, 21.05.2021 01:00