
Chemistry, 31.12.2019 06:31 feliciagraham14
The element x forms ions of charge +2 and the element y forms ions of charge -3. the most likely formula for a binary compound between these two elements is:

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1


Chemistry, 23.06.2019 03:30
Select the correct lewis structure for fluorine which is group 7a element?
Answers: 1
You know the right answer?
The element x forms ions of charge +2 and the element y forms ions of charge -3. the most likely for...
Questions


Mathematics, 05.05.2020 07:18

Mathematics, 05.05.2020 07:18

Mathematics, 05.05.2020 07:18




Biology, 05.05.2020 07:18


Mathematics, 05.05.2020 07:18

Arts, 05.05.2020 07:18


Social Studies, 05.05.2020 07:18





Mathematics, 05.05.2020 07:18


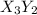 is the formula.
is the formula.

