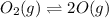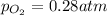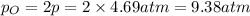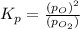Chemistry, 31.12.2019 04:31 angelespinosa521
Asample of o2(g) is placed in an otherwise empty, rigid container at 4224 k at an initial pressure of 4.97 atm, where it decomposes to o(g) by the reaction below.
o2(g) ⇄ 2 o(g)
at equilibrium, the partial pressure of o2 is 0.28 atm. calculate kp for this reaction at 4224 k.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:00
If a reaction has g of -136kj at 110°c, will it be spontaneous at this temperature (110°c)? yes or no
Answers: 2

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 08:30
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

You know the right answer?
Asample of o2(g) is placed in an otherwise empty, rigid container at 4224 k at an initial pressure o...
Questions

History, 23.09.2019 13:00

Mathematics, 23.09.2019 13:00

Chemistry, 23.09.2019 13:00


English, 23.09.2019 13:00






History, 23.09.2019 13:00

Mathematics, 23.09.2019 13:00

Chemistry, 23.09.2019 13:00

History, 23.09.2019 13:00

Mathematics, 23.09.2019 13:00



Biology, 23.09.2019 13:00


Mathematics, 23.09.2019 13:00

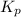 at 4224 K is 314.23.
at 4224 K is 314.23.