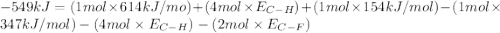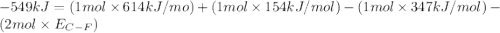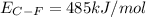Chemistry, 31.12.2019 02:31 yazanadel56
Consider the following reaction: c2h4(g) + f2(g) > c2h4f2(g) delta h = -549 kjestimate the carbon-fluorine bond energy given that the c-c bond energy is 347 kj/mol, the c=c bond energy is 614 kj/mol, and the f-f bond energy is 154 kj/mol.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2


Chemistry, 23.06.2019 03:30
In general metals get as you move from left to right across the periodic table.
Answers: 1
You know the right answer?
Consider the following reaction: c2h4(g) + f2(g) > c2h4f2(g) delta h = -549 kjestimate the carbo...
Questions


Mathematics, 30.10.2019 00:31


History, 30.10.2019 00:31

Mathematics, 30.10.2019 00:31

Mathematics, 30.10.2019 00:31

English, 30.10.2019 00:31

Mathematics, 30.10.2019 00:31






Mathematics, 30.10.2019 00:31





Computers and Technology, 30.10.2019 00:31

![\Delta H_{rxn}=\sum [n_{i}\times (E_{bond})_{i}]-\sum [n_{j}\times (E_{bond})_{j}]](/tpl/images/0437/8404/d74f8.png)
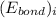 and
and 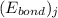 represents average bond energy in breaking "i" th bond and forming "j" th bond respectively.
represents average bond energy in breaking "i" th bond and forming "j" th bond respectively.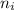 and
and 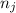 are number of moles of bond break and form respectively.
are number of moles of bond break and form respectively.