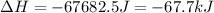Chemistry, 31.12.2019 01:31 cearadenney7067
How much energy must be removed from a 125 g sample of benzene (molar mass= 78.11 g/mol) at 425.0 k to liquify the sample and lower the temperature to 335.0 k? the following physical data may be useful.
δhvap = 33.9 kj/mol
δhfus = 9.8 kj/mol
cliq = 1.73 j/g°c
cgas = 1.06 j/g°c
csol = 1.51 j/g°c
tmelting = 279.0 k
tboiling = 353.0 k

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1
You know the right answer?
How much energy must be removed from a 125 g sample of benzene (molar mass= 78.11 g/mol) at 425.0 k...
Questions




Mathematics, 09.12.2020 20:50




Biology, 09.12.2020 20:50

Mathematics, 09.12.2020 20:50

English, 09.12.2020 20:50

History, 09.12.2020 20:50





Mathematics, 09.12.2020 20:50

Mathematics, 09.12.2020 20:50



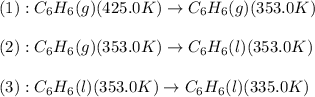
![\Delta H=[m\times c_{p,g}\times (T_{final}-T_{initial})]+m\times \Delta H_{vap}+[m\times c_{p,l}\times (T_{final}-T_{initial})]](/tpl/images/0437/7629/fdeb2.png)
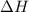 = heat released by the reaction = ?
= heat released by the reaction = ?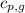 = specific heat of gaseous benzene =
= specific heat of gaseous benzene = 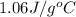
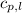 = specific heat of liquid benzene =
= specific heat of liquid benzene = 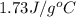
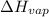 = enthalpy change for vaporization =
= enthalpy change for vaporization = 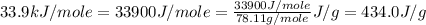
![\Delta H=[125g\times 1.06J/g.K\times (353.0-(425.0))K]+125g\times -434.0J/g+[125g\times 1.73J/g.K\times (335.0-353.0)K]](/tpl/images/0437/7629/08ed2.png)