
Chemistry, 31.12.2019 00:31 niescarlosj
The combustion of ammonia by the following reaction yields nitric oxide and water
4 nh3 (g) + 5 o2 (g) → 4 no (g) + 6 h2o (g)
determine the heat of reaction (δhrxn) for this reaction by using the following thermochemical data:
n2 (g) + o2 (g) → 2 no (g) δh = 180.6 kj
n2 (g) + 3 h2 (g) → 2 nh3 (g) δh = - 91.8 kj
2 h2 (g) + o2 (g) → 2 h2o (g) δh = - 483.7 kj
hess' law problems
2. determine the heat of reaction (δhrxn) for the process by which hydrazine (n2h4) is formed from its elements:
n2 (g) + 2 h2 (g) → n2h4 (g)
by using the following thermochemical data:
n2h4 (g) + o2 (g) → n2 (g) + 2 h2o (g) δh = - 622.2 kj
h2 (g) + 1/2 o2 (g) → h2o (g) δh = - 285.8 kj
hess' law problems
3. determine the heat of reaction (δhrxn) for the process by which lead (ii) chloride (pbcl2) is formed from its elements:
pb (s) + cl2 (g) → pbcl2 (s)
by using the following thermochemical data:
pb (s) + 2 cl2 (g) → pbcl4 (l) δh = - 329.3 kj
pbcl2 (s) + cl2 (g) → pbcl4 (l) δh = + 30.1 kj
hess' law problems
4. determine the heat of reaction (δhrxn) for the process by which acetic acid (ch3cooh) is formed from its elements:
c (graphite) + 2 h2 (g) + o2 (g) → ch3cooh (l)
by using the following thermochemical data:
ch3cooh (l) + o2 → 2 co2 (g) + 2 h2o(l) δh = - 871 kj
c (graphite) + o2 (g) → 2 co2 (g) δh = - 394 kj
h2 (s) + 1/2 o2 (g) → h2o(l) δh = - 286 kj
heat of formation problems
5. determine the heat of reaction (δhrxn) for the combustion of ethanol (c2h5oh) by using heat of formation data:
c2h5oh (l) + 3 o2 (g) → 2 co2 + 3 h2o (g)
heat of formation problems
6. determine the heat of reaction (δhrxn) for the chlorination of methane (ch4) by using heat of formation data:
ch4 (g) + 4 cl2 (g) → ccl4 (g) + 4 hcl (g)
heat of formation problems
7. determine the heat of reaction (δhrxn) for the reduction of acetaldehyde (ch3cho) to ethanol (c2h5oh) by using heat of formation data:
ch3cho (g) + h2 (g) → c2h5oh (l)
heat of formation problems
8. determine the heat of reaction (δhrxn) for the reaction of calcium carbonate (caco3) with hcl to produce co2 by using heat of formation data:
caco3 (s) + 2 hcl (g) → cacl2 (s) + co2 (g) + h2o (g)
expert answer

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Water (2510 g ) is heated until it just begins to boil. if the water absorbs 5.09×105 j of heat in the process, what was the initial temperature of the water?
Answers: 3

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1
You know the right answer?
The combustion of ammonia by the following reaction yields nitric oxide and water
4 nh3...
4 nh3...
Questions





English, 23.10.2019 03:00










Chemistry, 23.10.2019 03:00


History, 23.10.2019 03:00

Mathematics, 23.10.2019 03:00


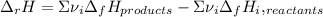
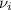 accounts for the stoichiometric coefficient of the ith compound based on the standard enthalpies of formation.
accounts for the stoichiometric coefficient of the ith compound based on the standard enthalpies of formation.




