
Chemistry, 30.12.2019 23:31 6224968918
Outline the steps needed to determine the limiting reactant when 30.0 g of propane, c3h8, is burned with 75.0 g of oxygen.
percent yield = 0.8347g / 0.9525 g × 100% = 87.6%

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
Which set of characteristics best describes igneous rock? a) largest type of rock, made of organic matter, hardest type of rock b) least abundant type of rock, made of other rocks, made mostly of minerals c) found on all continents, contains wavy bands of stripes, contains fossils d) most abundant type in earth's crust, made of magma/lava, contains no fossils
Answers: 1


Chemistry, 23.06.2019 01:00
Iron (fe) reacts with copper sulfate (cuso4) to form iron (ii) sulfate. in this reaction, cu2+ gains electrons to form cu. which statement is true about this reaction? fe(s) + cuso4(aq) → feso4(aq) + cu(s)
Answers: 3

You know the right answer?
Outline the steps needed to determine the limiting reactant when 30.0 g of propane, c3h8, is burned...
Questions

Mathematics, 14.04.2021 14:00

Physics, 14.04.2021 14:00



Mathematics, 14.04.2021 14:00



Health, 14.04.2021 14:00

Mathematics, 14.04.2021 14:00



Biology, 14.04.2021 14:00



Social Studies, 14.04.2021 14:00

Biology, 14.04.2021 14:00


Mathematics, 14.04.2021 14:00


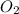
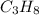 = 30.0 g
= 30.0 g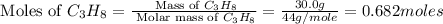
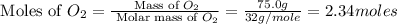

 moles of
moles of 

