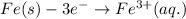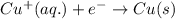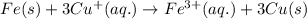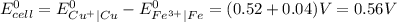Determine the overall reaction and its standard cell potential (in v) at 25°c for the reaction involving the galvanic cell made from a half-cell consisting of a copper electrode in 1 m copper(i) nitrate solution and a half-cell consisting of an iron electrode in 1 m iron(iii) nitrate. (include states-of-matter under the given conditions in your answer. use the lowest possible whole number coefficients.) overall reaction

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Why do sodium and neon have vastly different chemical and physical properties despite having similar atomic masses?
Answers: 2

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

You know the right answer?
Determine the overall reaction and its standard cell potential (in v) at 25°c for the reaction invol...
Questions


Mathematics, 29.09.2021 20:40

English, 29.09.2021 20:40


Biology, 29.09.2021 20:40

Mathematics, 29.09.2021 20:40

Chemistry, 29.09.2021 20:40

Mathematics, 29.09.2021 20:40

Mathematics, 29.09.2021 20:40

Physics, 29.09.2021 20:40


Chemistry, 29.09.2021 20:40

Mathematics, 29.09.2021 20:40

Mathematics, 29.09.2021 20:40

Mathematics, 29.09.2021 20:40

Chemistry, 29.09.2021 20:40


Arts, 29.09.2021 20:40


Physics, 29.09.2021 20:40

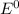 ) are given below-
) are given below-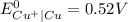
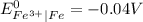
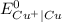 is greater than
is greater than 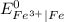 therefore Fe will be oxidized to
therefore Fe will be oxidized to 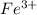 and
and 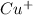 will be reduced to Cu
will be reduced to Cu