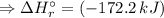Chemistry, 28.12.2019 05:31 lisamiller
Calculate horxn for the following reaction: h3aso4(aq) + 4 h2(g) --> ash3(g) + 4 h2o(l)(hof [ash3(g)] = 66.4 kj/mol; hof [h3aso4(aq)] = -904.6 kj/mol; hof [h2o(l)] = -285.8 kj/mol)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:40
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3

Chemistry, 22.06.2019 23:00
What is the mass of naoh that would have to be added to 500 ml of a solution of 0.20 m acetic acid in order to achieve a ph of 5.0?
Answers: 1

Chemistry, 23.06.2019 03:40
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh). ka(c6h3cooh) = 6.4 x 10^-5. what is the ph of the solution after the addition of 1 x 10^-3 moles of naoh? you may assume no volume change to the solution upon addition of the naoh.
Answers: 2
You know the right answer?
Calculate horxn for the following reaction: h3aso4(aq) + 4 h2(g) --> ash3(g) + 4 h2o(l)(hof [a...
Questions

English, 25.09.2019 12:30

Biology, 25.09.2019 12:30

Physics, 25.09.2019 12:30


Mathematics, 25.09.2019 12:30


History, 25.09.2019 12:30

Mathematics, 25.09.2019 12:30

History, 25.09.2019 12:30

Computers and Technology, 25.09.2019 12:30


Business, 25.09.2019 12:30



Computers and Technology, 25.09.2019 12:30

Business, 25.09.2019 12:30

Mathematics, 25.09.2019 12:30


Spanish, 25.09.2019 12:30

Social Studies, 25.09.2019 12:30

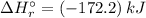
![\Delta H_{f}^{\circ } [H_{3}AsO_{4}(aq)]](/tpl/images/0435/4880/bc889.png) = -904.6 kJ/mol
= -904.6 kJ/mol![\Delta H_{f}^{\circ } [H_{2}(g)]](/tpl/images/0435/4880/b83d7.png) = 0 kJ/mol,
= 0 kJ/mol,![\Delta H_{f}^{\circ } [AsH_{3}(g)]](/tpl/images/0435/4880/eaaf4.png) = +66.4 kJ/mol
= +66.4 kJ/mol![\Delta H_{f}^{\circ } [H_{2}O(l)]](/tpl/images/0435/4880/46f61.png) = -285.8 kJ/mol
= -285.8 kJ/mol 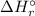 = ?
= ?
![\Delta H_{r}^{\circ } = [1 \times \Delta H_{f}^{\circ } [AsH_{3} (g)] + 4 \times \Delta H_{f}^{\circ } [H_{2}O(l)]] - [1 \times \Delta H_{f}^{\circ } [H_{3}AsO_{4}(aq)] + 4 \times \Delta H_{f}^{\circ } [H_{2}(g)]](/tpl/images/0435/4880/cee8f.png)
![\Rightarrow \Delta H_{r}^{\circ } = [1 \times (+66.4\,kJ/mol) + 4 \times (-285.8\,kJ/mol) ] - [1 \times (-904.6\,kJ/mol) + 4 \times (0\,kJ/mol)]](/tpl/images/0435/4880/49bdc.png)
![\Rightarrow \Delta H_{r}^{\circ } = [-1076.8\, kJ] - [-904.6\,kJ]](/tpl/images/0435/4880/a7183.png)