
Chemistry, 28.12.2019 05:31 nkazmirski5598
Consider a voltaic cell where the anode half-reaction is zn(s) → zn2+(aq) + 2 e− and the cathode half-reaction is sn2+(aq) + 2 e– → sn(s). what is the concentration of sn2+ if zn2+ is 2.5 × 10−3 m and the cell emf is 0.660 v? the standard reduction potentials are given below
zn+2(aq) + 2 e−→ zn(s) e∘red = −0.76 v
sn2+(aq) + 2 e– →sn(s) e∘red =-0.136 v
a. 9.0*10^-3 m
b. 3..3*10^-2 m
c. 6.9*10^-4 m
d. 7.6*10^-3 m

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1

Chemistry, 23.06.2019 01:00
Wind and moving water provide energy. chemical mechanical thermal none of the above
Answers: 1
You know the right answer?
Consider a voltaic cell where the anode half-reaction is zn(s) → zn2+(aq) + 2 e− and the cathode hal...
Questions



History, 10.02.2020 20:00





Computers and Technology, 10.02.2020 20:00

Physics, 10.02.2020 20:00


SAT, 10.02.2020 20:00



Health, 10.02.2020 20:00

Mathematics, 10.02.2020 20:00

Business, 10.02.2020 20:00

Chemistry, 10.02.2020 20:00


English, 10.02.2020 20:00

Biology, 10.02.2020 20:00


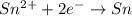
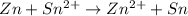
 :
:![E_{cell}=(E_{Sn^{2+}\mid Sn}^{0}-E_{Zn^{2+}\mid Zn}^{0})-\frac{0.059}{n}log\frac{[Zn^{2+}]}{[Sn^{2+}]}](/tpl/images/0435/4933/a904f.png)
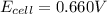 and
and ![[Zn^{2+}]=2.5\times 10^{-3}M](/tpl/images/0435/4933/eef66.png)
![0.660V=(-0.136V+0.76V)-\frac{0.059}{2}log\frac{2.5\times 10^{-3}M}{[Sn^{2+}]}](/tpl/images/0435/4933/821cc.png)
![[Sn^{2+}]=4.2\times 10^{-2}M](/tpl/images/0435/4933/6b94f.png)
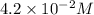 is most closest to
is most closest to 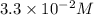 therefore option (B) is correct.
therefore option (B) is correct.

