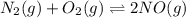Chemistry, 28.12.2019 04:31 narutoxptheninja
For the reactionn2(g)+o2(g)? 2no(g)classify each of the following actions by whether it causes a leftward shift, a rightward shift, or no shift in the direction of the reaction. a) half oxygenb) double oxygenc) double nitrogend) half nitrogene) double nitrogen monoxidef) half nitrogen monoxide

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1
You know the right answer?
For the reactionn2(g)+o2(g)? 2no(g)classify each of the following actions by whether it causes a lef...
Questions

Social Studies, 18.11.2020 02:40


Mathematics, 18.11.2020 02:40


Mathematics, 18.11.2020 02:40


Law, 18.11.2020 02:40


Mathematics, 18.11.2020 02:40





Business, 18.11.2020 02:40

Mathematics, 18.11.2020 02:40

Mathematics, 18.11.2020 02:40



History, 18.11.2020 02:40

Mathematics, 18.11.2020 02:40