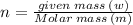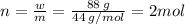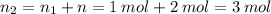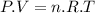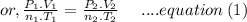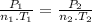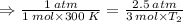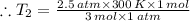Chemistry, 28.12.2019 04:31 hilljade45
Aflask of fixed volume contains 1.0 mole of gaseous carbon dioxide and 88 g of solid carbon dioxide. the original pressure and temperature in the flask is 1.0 atm and 300. k. all of the solid carbon dioxide sublimes. the final pressure in the flask is 2.5 atm. what is the final temperature? assume the solid carbon dioxide takes up negligible volume.
a. 150 k
b. 200 k
c. 250 k
d. 300 k
e. 400 k

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

You know the right answer?
Aflask of fixed volume contains 1.0 mole of gaseous carbon dioxide and 88 g of solid carbon dioxide....
Questions





Physics, 07.01.2021 01:00

Mathematics, 07.01.2021 01:00

Mathematics, 07.01.2021 01:00


Biology, 07.01.2021 01:00

Geography, 07.01.2021 01:00

Spanish, 07.01.2021 01:00




Mathematics, 07.01.2021 01:00



Biology, 07.01.2021 01:00

History, 07.01.2021 01:00