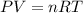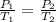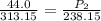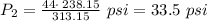Chemistry, 28.12.2019 03:31 brooke0713
The pressure inside an automobile tire is 44.0 pounds per square inch (psi) on a very warm summer day (40.0 °c). calculate the tire pressure in the same tire on a very cold winter day (-35.0 °c) five months later, assuming that no air escapes from the tire. hint: you will need to make another reasonable assumption that is not stated here.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:10
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3


Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2
You know the right answer?
The pressure inside an automobile tire is 44.0 pounds per square inch (psi) on a very warm summer da...
Questions

Mathematics, 19.05.2021 19:50




Mathematics, 19.05.2021 19:50


Mathematics, 19.05.2021 19:50





History, 19.05.2021 19:50



Mathematics, 19.05.2021 19:50

Biology, 19.05.2021 19:50

History, 19.05.2021 19:50


Geography, 19.05.2021 19:50

Mathematics, 19.05.2021 19:50