
Chemistry, 28.12.2019 02:31 Mercedes12152002
For the reaction x + y → z, the reaction rate is found to depend only upon the concentration of x. a plot of 1/x verses time gives a straight line. picture what is the rate law for this reaction?
a. rate = k [x]
b. rate = k [x]2
c. rate = k [x][y]
d. rate = k [x]2[y]

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:10
Harvey mixes two liquids. which observation of the new mixture most likely indicates a precipitate is forming?
Answers: 2

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2
You know the right answer?
For the reaction x + y → z, the reaction rate is found to depend only upon the concentration of x. a...
Questions



Social Studies, 26.10.2020 20:00

Mathematics, 26.10.2020 20:00

Biology, 26.10.2020 20:00

Mathematics, 26.10.2020 20:00

Mathematics, 26.10.2020 20:00


Mathematics, 26.10.2020 20:00


Mathematics, 26.10.2020 20:00

Mathematics, 26.10.2020 20:00



History, 26.10.2020 20:00

History, 26.10.2020 20:00


History, 26.10.2020 20:00

Mathematics, 26.10.2020 20:00

Mathematics, 26.10.2020 20:00

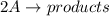
![rate = k[A]^{2}](/tpl/images/0435/2384/bea1f.png) , where k is rate constant and [A] is concentration of A
, where k is rate constant and [A] is concentration of A![\frac{1}{[A]}=\frac{1}{[A]_{0}}+kt](/tpl/images/0435/2384/a6d73.png) , where
, where ![[A]_{0}](/tpl/images/0435/2384/48818.png) is initial concentration of reactant A and [A] is concentration of A after "t" time (k and [A]_{0}[/tex] are constants)
is initial concentration of reactant A and [A] is concentration of A after "t" time (k and [A]_{0}[/tex] are constants)![\frac{1}{[A]}](/tpl/images/0435/2384/153b1.png) is y and t is x.
is y and t is x.![rate = k[X]^{2}](/tpl/images/0435/2384/7b96c.png)


