
Chemistry, 28.12.2019 00:31 quincyjosiah07
How much heat is absorbed/released when 20.00 g of nh3(g) reacts in the presence of excess o2 (g) to produce no (g) and h2o (l) according to the following chemical equation? 4nh3 (g) + 5o2 (g) > 4no (g) +6h2o (l) δ h: +1168 kja. 342.9 kj of heat are absorbed. b. 342.9 kj of heat are released. c. 1372 kj of heat are absorbed. d. 1372 kj of heat are released.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1

Chemistry, 23.06.2019 00:00
How many peaks will be present in a mass spectrum for brcl?
Answers: 1

Chemistry, 23.06.2019 00:30
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1

Chemistry, 23.06.2019 01:00
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2
You know the right answer?
How much heat is absorbed/released when 20.00 g of nh3(g) reacts in the presence of excess o2 (g) to...
Questions













Advanced Placement (AP), 18.09.2019 21:10







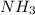 as:-
as:-
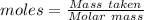
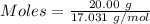
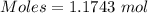
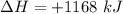
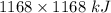 of heat
of heat

