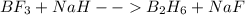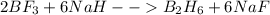Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:30
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2

Chemistry, 23.06.2019 00:00
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1

Chemistry, 23.06.2019 09:20
1) a. water molecule breaks up into hydrogen and oxygen on passing electricity. does this involve breaking intermolecular or intramolecular forces of attraction. explain b. on boiling water changes to water vapor. does this involve breaking intermolecular or intramolecular forces of attraction. explain methanol evaporates faster than water. contrast the intermolecular forces and the vapor pressures of methanol and water?
Answers: 2

Chemistry, 23.06.2019 16:00
Be sure to answer all parts. the catalytic destruction of ozone occurs via a two-step mechanism, where x can be any of several species: (1) x + o3 → xo + o2 [slow] (2) xo + o → x + o2 [fast] (a) write the overall reaction. o3 + o → 2 o2 o3 + xo → x + 2 o2 xo + x + o3 + o → xo + x + 2 o2 x + o3 → xo + o2 (b) write the rate law for each step (using k for the rate constant). reaction 1: reaction 2: k[x][o2] k[xo][o2] k[x][o3] k[xo][o3] k[xo][o2] k[x][o] k[x][o2] k[xo][o] (c) x acts as a and xo acts as a catalyst intermediate intermediate catalyst (d) high-flying aircraft release no into the stratosphere, which catalyzes this process. when o3 and no concentrations are 3 ă— 1012 molecule/cm3 and 9.9 ă— 109 molecule/cm3, respectively, what is the rate of o3 depletion? the rate constant k for the rate-determining step is 6 ă— 10â’15 (cm3)2/moleculeâ·s. give your answer in scientific notation. use one significant figure in your answer. ă— 10 molecule/s
Answers: 2
You know the right answer?
For the skeletal chemical equation bf3(g) + nah(s) → b2h6(g) + naf(s) what is the coefficient of b2h...
Questions


Mathematics, 30.05.2021 19:30


Mathematics, 30.05.2021 19:30

English, 30.05.2021 19:30


Mathematics, 30.05.2021 19:30


Mathematics, 30.05.2021 19:30


English, 30.05.2021 19:30






Mathematics, 30.05.2021 19:30

Mathematics, 30.05.2021 19:30

English, 30.05.2021 19:30

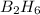 in the balanced equation is 1.
in the balanced equation is 1.