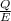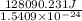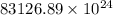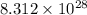Chemistry, 27.12.2019 05:31 MiddleSchool2005
Acontainer with 0.389 l of water is placed in a microwave and radiated with electromagnetic energy with a wavelength of 12.9 cm. the temperature of the water rose by 78.7 °c. calculate the number of photons that were absorbed by the water. assume water has a density of 1.00 g ⋅ ml − 1 and a specific heat of 4.184 j ⋅ g − 1 ⋅ ° c − 1 .

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2


Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

Chemistry, 22.06.2019 22:00
11) burning your hand when accidentally touching a hot plate is an example of which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 2
You know the right answer?
Acontainer with 0.389 l of water is placed in a microwave and radiated with electromagnetic energy w...
Questions

Mathematics, 21.10.2020 14:01


Mathematics, 21.10.2020 14:01




Mathematics, 21.10.2020 14:01


Biology, 21.10.2020 14:01

Mathematics, 21.10.2020 14:01

Physics, 21.10.2020 14:01


Biology, 21.10.2020 14:01

Social Studies, 21.10.2020 14:01

Social Studies, 21.10.2020 14:01

Computers and Technology, 21.10.2020 14:01

Mathematics, 21.10.2020 14:01



Biology, 21.10.2020 14:01

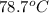
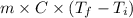
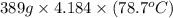
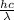
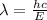
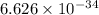 J s
J s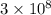 m/s
m/s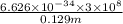
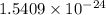 J/photons
J/photons