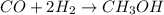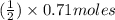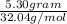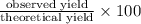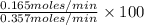Chemistry, 27.12.2019 05:31 lorelei7668
Methanol (ch3oh) can be produced by the following reaction: co(g) 1 2h2(g) 88n ch3oh(g) hydrogen at stp flows into a reactor at a rate of 16.0 l/min. carbon monoxide at stp flows into the reactor at a rate of 25.0 l/min. if 5.30 g of methanol is produced per minute, what is the percent yield of the reaction?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 21:00
Acandle’s wick is the fabric string that holds the flame, and it burns down at a constant slow pace when the candle is lit. the wick is usually surrounded by wax. which is the most important property of covalent compounds that makes them useful for making candle wax? a low boiling point a low melting point a high boiling point a high melting point
Answers: 1

Chemistry, 23.06.2019 05:00
Which of the following describes qualitative data? a) recording the temperature of a solid as it is warmed. b) noting the color of a solution as it is heated. c) measuring the volume of an object by water displacement. d) taking the mass of an object using a balance.
Answers: 2

You know the right answer?
Methanol (ch3oh) can be produced by the following reaction: co(g) 1 2h2(g) 88n ch3oh(g) hydrogen at...
Questions

Physics, 24.06.2019 17:00





Mathematics, 24.06.2019 17:00

Mathematics, 24.06.2019 17:00

Mathematics, 24.06.2019 17:00

Computers and Technology, 24.06.2019 17:00


Mathematics, 24.06.2019 17:00





History, 24.06.2019 17:00


Computers and Technology, 24.06.2019 17:00

Social Studies, 24.06.2019 17:00

Mathematics, 24.06.2019 17:00

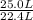 per minute
per minute
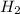 being fed per minute =
being fed per minute = 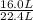 per minute
per minute