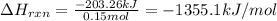Chemistry, 27.12.2019 04:31 liljohnsjs218
Asample of ethanol (c2h5oh), weighing 6.83 g underwent combustion in a bomb calorimeter by the following reaction: c2h5oh (l) + 3 o2 (g) → 2 co2 (g) + 3 h2o (l)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3

Chemistry, 22.06.2019 23:00
What extra step distinguishes fermentation from glycolysis
Answers: 1
You know the right answer?
Asample of ethanol (c2h5oh), weighing 6.83 g underwent combustion in a bomb calorimeter by the follo...
Questions

History, 18.11.2019 18:31



Arts, 18.11.2019 18:31






Biology, 18.11.2019 18:31



English, 18.11.2019 18:31

History, 18.11.2019 18:31

Mathematics, 18.11.2019 18:31


Mathematics, 18.11.2019 18:31



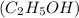 , weighing 6.83 g underwent combustion in a bomb calorimeter by the following reaction:
, weighing 6.83 g underwent combustion in a bomb calorimeter by the following reaction: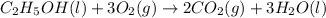
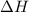 of the reaction?
of the reaction?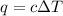
 = change in temperature =
= change in temperature = 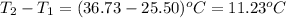
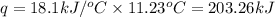
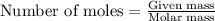
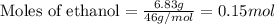
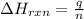
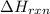 = enthalpy change of the reaction
= enthalpy change of the reaction