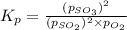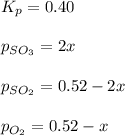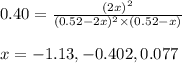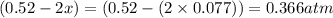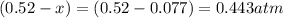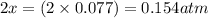Chemistry, 27.12.2019 03:31 IIHarmonyII
At 920 k, kp = 0.40 for the following reaction. 2 so2(g) + o2(g) equilibrium reaction arrow 2 so3(g) calculate the equilibrium partial pressures of so2, o2, and so3 produced from an initial mixture in which the partial pressures of so2 and o2 = 0.52 atm and the partial pressure of so3 = 0 (exactly).

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 00:30
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1
You know the right answer?
At 920 k, kp = 0.40 for the following reaction. 2 so2(g) + o2(g) equilibrium reaction arrow 2 so3(g)...
Questions

Mathematics, 25.02.2021 23:40


Mathematics, 25.02.2021 23:40





English, 25.02.2021 23:40


Biology, 25.02.2021 23:40




Mathematics, 25.02.2021 23:40



Business, 25.02.2021 23:40

Mathematics, 25.02.2021 23:40


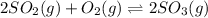
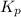 for above equation follows:
for above equation follows: