

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which is the layer underground where all empty spaces are filled with a combination of air and water ?
Answers: 1

Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1

Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1
You know the right answer?
How many g of aluminum are needed to react completely with 4.40 gram of mn304, according to the chem...
Questions












Mathematics, 05.05.2020 17:08







Mathematics, 05.05.2020 17:08

Mathematics, 05.05.2020 17:08

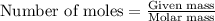 .....(1)
.....(1)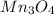 = 4.40 g
= 4.40 g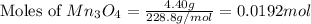
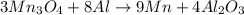
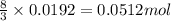 of aluminium
of aluminium


