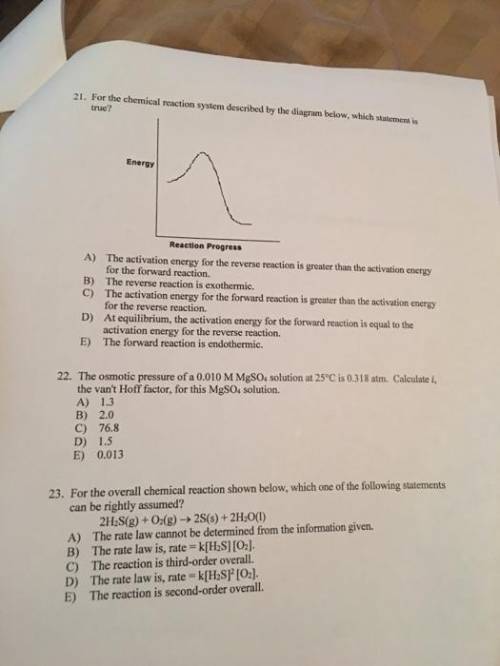
Chemistry, 27.12.2019 00:31 alexisthegirl
For the chemical reaction system described by the diagram below, which statement is true? picture the forward reaction is endothermic. the activation energy for the forward reaction is greater than the activation energy for the reverse reaction. at equilibrium, the activation energy for the forward reaction is equal to the activation energy for the reverse reaction. the activation energy for the reverse reaction is greater than the activation energy for the forward reaction. the reverse reaction is exothermic.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
The wave shown on the electromagnetic spectrum disturb the medium it passes through a)different frequency. b)the same frequency .
Answers: 2

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 23.06.2019 12:00
In ccl4 is there a certain length that the main atom carbon is a certain distance from the chlorine’s?
Answers: 1
You know the right answer?
For the chemical reaction system described by the diagram below, which statement is true? picture t...
Questions

Mathematics, 08.01.2021 20:10




Arts, 08.01.2021 20:10

Mathematics, 08.01.2021 20:10

Mathematics, 08.01.2021 20:10

English, 08.01.2021 20:10


Mathematics, 08.01.2021 20:10

Mathematics, 08.01.2021 20:10



Mathematics, 08.01.2021 20:10

Mathematics, 08.01.2021 20:10

Mathematics, 08.01.2021 20:10

Biology, 08.01.2021 20:10

Mathematics, 08.01.2021 20:10


Physics, 08.01.2021 20:10