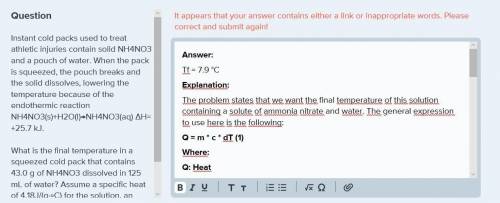
Instant cold packs used to treat athletic injuries contain solid nh4no3 and a pouch of water. when the pack is squeezed, the pouch breaks and the solid dissolves, lowering the temperature because of the endothermic reaction nh4no3(s)+h2o(l)→nh4no3(aq) δh= +25.7 kj.
what is the final temperature in a squeezed cold pack that contains 43.0 g of nh4no3 dissolved in 125 ml of water? assume a specific heat of 4.18j/(g⋅∘c) for the solution, an initial temperature of 27.5 ∘c, and no heat transfer between the cold pack and the environment.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:20
Determine which intermolecular forces are the dominant (strongest) forces for a pure sample of each of the following molecules by placing the molecules into the correct bins. drag the appropriate molecular formula to their respective bins.
Answers: 3

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3
You know the right answer?
Instant cold packs used to treat athletic injuries contain solid nh4no3 and a pouch of water. when t...
Questions


Mathematics, 12.02.2021 18:00




History, 12.02.2021 18:00


Mathematics, 12.02.2021 18:00

Geography, 12.02.2021 18:00

Mathematics, 12.02.2021 18:00


Mathematics, 12.02.2021 18:00



Social Studies, 12.02.2021 18:00

Computers and Technology, 12.02.2021 18:00

Mathematics, 12.02.2021 18:00

Mathematics, 12.02.2021 18:00

Physics, 12.02.2021 18:00

Chemistry, 12.02.2021 18:00