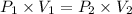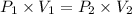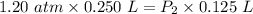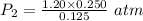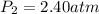Chemistry, 26.12.2019 21:31 makaylahunt
Asample of neon gas at 1.20 atm compresses from 0.250 l to 0.125 l. if the temperature remains constant, what is the final pressure in atm? a. 0.600 atm b. 1.000 atm c. 1.20 atm d. 2.40 atm e. none of the above

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 01:30
Pls! plant cells and animal cells were observed under a microscope. the characteristics of two cells are listed below. cell p: does not capture sunlight cell q: has cytoplasm but no chloroplast which statement about the two cells is correct? cell q also has a cell wall. cell q also has large vacuole. cell p also has a large vacuole. cell p also has a cell membrane.
Answers: 1

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1
You know the right answer?
Asample of neon gas at 1.20 atm compresses from 0.250 l to 0.125 l. if the temperature remains const...
Questions



Physics, 17.04.2020 20:41


Biology, 17.04.2020 20:41

History, 17.04.2020 20:41






Mathematics, 17.04.2020 20:41

Mathematics, 17.04.2020 20:41





Mathematics, 17.04.2020 20:41

Biology, 17.04.2020 20:41