

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2
You know the right answer?
Using standard heats of formation found in the appendix in your textbook, what is δh∘rxn for the fol...
Questions



History, 25.08.2021 19:40

Mathematics, 25.08.2021 19:40




Mathematics, 25.08.2021 19:40

Mathematics, 25.08.2021 19:40

Mathematics, 25.08.2021 19:40

Mathematics, 25.08.2021 19:40


Computers and Technology, 25.08.2021 19:40



Mathematics, 25.08.2021 19:40



History, 25.08.2021 19:40

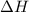
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f_{(product)}]-\sum [n\times \Delta H^o_f_{(reactant)}]](/tpl/images/0433/8700/72c39.png)
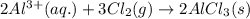
![\Delta H^o_{rxn}=[(2\times \Delta H^o_f_{(AlCl_3(s))})]-[(2\times \Delta H^o_f_{(Al^{3+}(aq.))})+(3\times \Delta H^o_f_{(Cl_2(g))})]](/tpl/images/0433/8700/164b7.png)
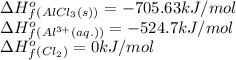
![\Delta H^o_{rxn}=[(2\times (-705.63))]-[(2\times (-524.7))+(3\times (0))]\\\\\Delta H^o_{rxn}=-361.86kJ](/tpl/images/0433/8700/c0739.png)


