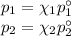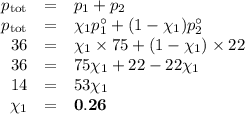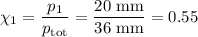Chemistry, 25.12.2019 03:31 vlactawhalm29
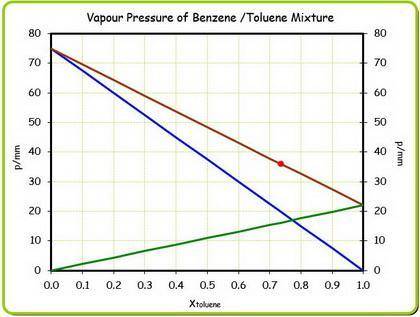
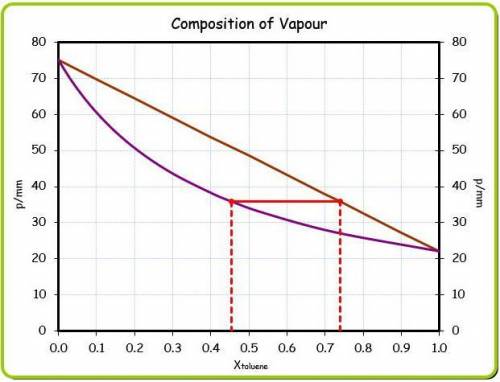
At 20 ? c the vapor pressure of benzene (c6h6)is 75 torr, and that of toluene (c7h8) is 22 torr
. assume that benzene and toluene form an ideal solution.
what is the composition in mole fractions of a solution that has a vapor pressure of 36torr at 20 degrees c?
what is the mole fraction of benzene in the vapor above the solution described in part (a)?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 12:30
A__ is two or more substances that are together in the same place but are not chemically combined
Answers: 1

Chemistry, 21.06.2019 14:40
Water ionizes by the equation h2o(l)⇌h+(aq)+oh−(aq) the extent of the reaction is small in pure water and dilute aqueous solutions. this reaction creates the following relationship between [h+] and [oh−]: kw=[h+][oh−] keep in mind that, like all equilibrium constants, the value of kw changes with temperature.
Answers: 1


Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3
You know the right answer?
At 20 ? c the vapor pressure of benzene (c6h6)is 75 torr, and that of toluene (c7h8) is 22 torr
Questions


Mathematics, 03.06.2021 05:30




French, 03.06.2021 05:30



Mathematics, 03.06.2021 05:40



Mathematics, 03.06.2021 05:40


Business, 03.06.2021 05:40


Mathematics, 03.06.2021 05:40