Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1

Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 22:30
What methods could you use to solubilize calcium carbonate
Answers: 1
You know the right answer?
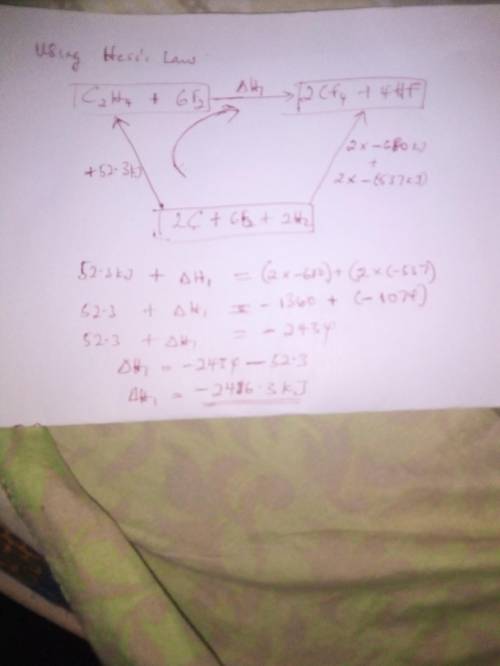
From the enthalpies of reactionh2 (g) + f2 (g) → 2 hf (g) δh = -537 kjc (s) + 2 f2 (g) → cf4 (g) δh...
Questions



Mathematics, 22.09.2020 17:01



Mathematics, 22.09.2020 17:01

English, 22.09.2020 17:01








Geography, 22.09.2020 17:01

Mathematics, 22.09.2020 17:01

English, 22.09.2020 17:01