
Chemistry, 25.12.2019 01:31 boneyke3720
The clectron in a hydrogen atom, originally in level n=9, undergoes a transition to a lower level by emitting a photon
of wavelength 384 nm. what is the final level of the electron?
c = 3.00 x 10 m/s,
h = 6.626 x 10^-34j-s,
rh= 2.179 x 10^-18

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

You know the right answer?
The clectron in a hydrogen atom, originally in level n=9, undergoes a transition to a lower level by...
Questions



Health, 31.08.2019 10:30


Mathematics, 31.08.2019 10:30




Chemistry, 31.08.2019 10:30

Social Studies, 31.08.2019 10:30

Chemistry, 31.08.2019 10:30

Mathematics, 31.08.2019 10:30

Geography, 31.08.2019 10:30


History, 31.08.2019 10:30

History, 31.08.2019 10:30

Chemistry, 31.08.2019 10:30

Health, 31.08.2019 10:30

English, 31.08.2019 10:30

Mathematics, 31.08.2019 10:30

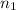 = 9,
= 9, 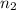 = ?
= ?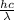
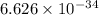 Js
Js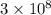 m/s
m/s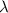 = wavelength
= wavelength ![\Delta E = -2.179 \times 10^{-18} J \times (Z)^{2}[\frac{1}{n^{2}_{2}} - \frac{1}{n^{2}_{1}}]](/tpl/images/0432/3307/57fbb.png)
![-2.179 \times 10^{-18} J \times (Z)^{2}[\frac{1}{n^{2}_{2}} - \frac{1}{n^{2}_{1}}]](/tpl/images/0432/3307/1f28d.png)
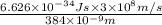 =
= ![-2.179 \times 10^{-18} J \times (1)^{2}[\frac{1}{n^{2}_{2}} - \frac{1}{(9)^{2}}]](/tpl/images/0432/3307/e4713.png)


