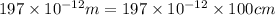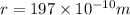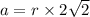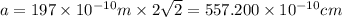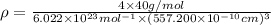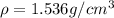Chemistry, 25.12.2019 00:31 joycetleiji1
Calcium has a cubic closest packed structure (fcc) as a solid. assuming that calcium has an atomic radius of 197 pm, calculate the density of solid calcium. (1 pm = 10-12 m, 100 cm = 1 m)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3


Chemistry, 22.06.2019 19:40
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests.which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3

You know the right answer?
Calcium has a cubic closest packed structure (fcc) as a solid. assuming that calcium has an atomic r...
Questions


Mathematics, 29.09.2019 03:00





Physics, 29.09.2019 03:00

Advanced Placement (AP), 29.09.2019 03:00


Biology, 29.09.2019 03:00


Social Studies, 29.09.2019 03:00



History, 29.09.2019 03:00

Chemistry, 29.09.2019 03:00




Biology, 29.09.2019 03:00

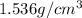 is the density of solid calcium.
is the density of solid calcium.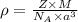
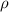 = density
= density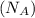 = Avogadro's number
= Avogadro's number