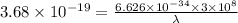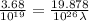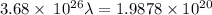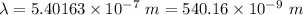Chemistry, 24.12.2019 21:31 juansantos7b
Aphoton with 2.3 ev of energy can eject an electron from potassium. what is the corresponding wavelength of this type of light? answer in nm. 1 ev = 1.60 x 10-19 j speed of light = 3.0 x 108 m/s planck's constant = 6.626 x 10-34 js socratic.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3


Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

Chemistry, 23.06.2019 02:30
What role does weathering have in shaping earth’s surface? a) it allows sediments to fall out of a medium. b) it sediments settle on a new surface. c) it breaks down older material into sediments. d) it transports sediments to a different location. will give brainliest, answer quickly.
Answers: 2
You know the right answer?
Aphoton with 2.3 ev of energy can eject an electron from potassium. what is the corresponding wavele...
Questions

Mathematics, 11.12.2020 03:40




English, 11.12.2020 03:40




History, 11.12.2020 03:40

Mathematics, 11.12.2020 03:40

Mathematics, 11.12.2020 03:40


History, 11.12.2020 03:40

Mathematics, 11.12.2020 03:40



History, 11.12.2020 03:40

Spanish, 11.12.2020 03:40

Chemistry, 11.12.2020 03:40

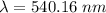
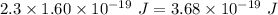
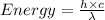
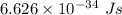
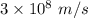
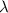 is the wavelength of the light being bombarded
is the wavelength of the light being bombarded