Chemistry, 24.12.2019 20:31 smartgirl61987
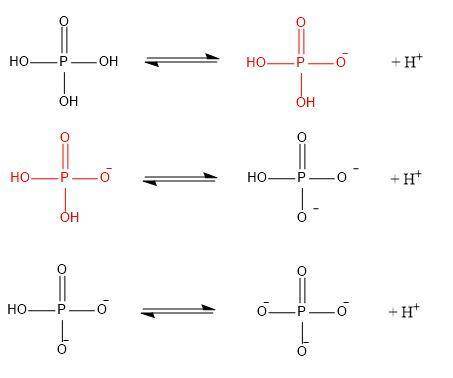
Phosphate is derived from the titration of phosphoric acid, h3po4. the three pka’s of h3po4 are 2.15, 7.20, and 12.35 respectively.
a/ write out the series of ionization (equilibrium) reactions corresponding to each ionization, making sure to write out the (flat) structure each molecule/ion as you do so. mark correct chemical bonds.
b/ in your diagram above, circle the dominant form of phosphate as it would appear at ph 5.7.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1
You know the right answer?
Phosphate is derived from the titration of phosphoric acid, h3po4. the three pka’s of h3po4 are 2.15...
Questions


Mathematics, 25.03.2021 18:00





English, 25.03.2021 18:00

Computers and Technology, 25.03.2021 18:00

Mathematics, 25.03.2021 18:00

Chemistry, 25.03.2021 18:00

Advanced Placement (AP), 25.03.2021 18:00

English, 25.03.2021 18:00

Biology, 25.03.2021 18:00

Chemistry, 25.03.2021 18:00






Biology, 25.03.2021 18:00